Skip over navigation
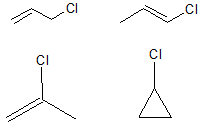
The first part of this question is a gentle introduction to the drawing of isomers. Structural isomers are those which involve different arrangements of the carbon skeleton, and different placements of the functional groups. It should be noted that cis and trans isomers are classed as geometric isomers, and so are not included here:
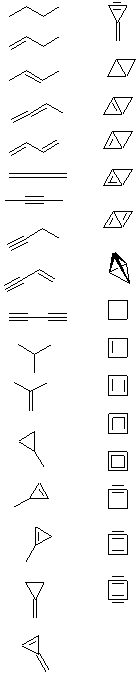
The next part of the question is deliberately open ended. We have been able to find just over 30 different structures (maybe you can find more?), although it is important to note that many of these structures would be too unstable to be formed in the lab. However, this question is an exercise in logical structural drawing, rather than a detailed analysis of chemical stability.
The IR spectrum has three clear peaks:
~3100 cm$^-1$ broad peak correspond to -OH
~3300 cm$^-1$ corresponds to -NH
~1700 cm$^-1$ corresponds to C$=$O, rather than C$=$C since the absorption is SHARP.
The important point to note is that the peak at 3300cm$^-1$ is only single rather than a double absorption, which corresponds to an NH but NOT NH$_2$.
The zoomed IR spectrum shows an absorption at ~1670cm$^-1$, which indicates that the molecule contains an amide group. Thus because of the single peak at 3300cm$^-1$, this is a secondary amide:
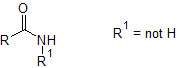
The NRM spectrum has 6 peaks, which shows that there are 6 different carbon environments. Since six of the eight carbon atoms are present in a benzene ring, the location of the substituents around the ring must be:
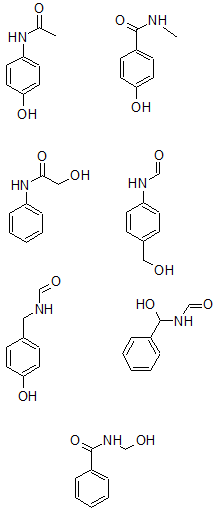
From all the data so far, seven possible structures are:
The m/z of 58 corresponds to $[C_2H_4NO]^+$.
The m/z of 93 corresponds to $[C_6H_5O]^+$.
The additional information that the molecule was constructed from a nitro-aryl suggests that the nitrogen must be attached directly to the benzene ring. The only structure of the three remaining is:
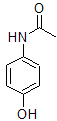
This molecule is paracetamol.
Or search by topic
Number and algebra
Geometry and measure
Probability and statistics
Working mathematically
Advanced mathematics
For younger learners
CSI: Chemical Scene Investigation
Age 16 to 18
Challenge Level 





- Problem
- Getting Started
- Student Solutions
The first part of this question is a gentle introduction to the drawing of isomers. Structural isomers are those which involve different arrangements of the carbon skeleton, and different placements of the functional groups. It should be noted that cis and trans isomers are classed as geometric isomers, and so are not included here:

The next part of the question is deliberately open ended. We have been able to find just over 30 different structures (maybe you can find more?), although it is important to note that many of these structures would be too unstable to be formed in the lab. However, this question is an exercise in logical structural drawing, rather than a detailed analysis of chemical stability.

The IR spectrum has three clear peaks:
~3100 cm$^-1$ broad peak correspond to -OH
~3300 cm$^-1$ corresponds to -NH
~1700 cm$^-1$ corresponds to C$=$O, rather than C$=$C since the absorption is SHARP.
The important point to note is that the peak at 3300cm$^-1$ is only single rather than a double absorption, which corresponds to an NH but NOT NH$_2$.
The zoomed IR spectrum shows an absorption at ~1670cm$^-1$, which indicates that the molecule contains an amide group. Thus because of the single peak at 3300cm$^-1$, this is a secondary amide:
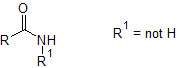
The NRM spectrum has 6 peaks, which shows that there are 6 different carbon environments. Since six of the eight carbon atoms are present in a benzene ring, the location of the substituents around the ring must be:

From all the data so far, seven possible structures are:

The m/z of 58 corresponds to $[C_2H_4NO]^+$.
The m/z of 93 corresponds to $[C_6H_5O]^+$.
The additional information that the molecule was constructed from a nitro-aryl suggests that the nitrogen must be attached directly to the benzene ring. The only structure of the three remaining is:

This molecule is paracetamol.
You may also like
The Mathematical Problems Faced by Advanced STEM Students
STEM students at university often encounter mathematical difficulties. This articles highlights the various content problems and the 7 key process problems encountered by STEM students.

