Skip over navigation
The most densely-packed planes for FCC are along the diagonals of the faces of the cube. Thus if the side length is $a$, $\sqrt{2}a = 4\mbox{ radii} \therefore a = \frac{4}{\sqrt{2}}r$. So the fill is $4\mbox{ atoms}/a^3 = \frac{\sqrt{2}\pi}{6} = 74\%\;.$
For BCC, the most densely-packed plane is the inside diagonal, $\therefore \sqrt{3}a = 4\mbox{ radii} \therefore a = \frac{4}{\sqrt{2}} r$. So the fill is $2\mbox{ atoms}/a^3 = \frac{\sqrt{3}\pi}{8} = 68\%\;.$
If you find the FCC side length in terms of the atomic radius of copper, you will find that the answer is very close to the experimentally-found density of copper, $8920\textrm{ kg/m}^3.$


Or search by topic
Number and algebra
Geometry and measure
Probability and statistics
Working mathematically
Advanced mathematics
For younger learners
Pack Man
Age 16 to 18
Challenge Level 





- Problem
- Student Solutions
The most densely-packed planes for FCC are along the diagonals of the faces of the cube. Thus if the side length is $a$, $\sqrt{2}a = 4\mbox{ radii} \therefore a = \frac{4}{\sqrt{2}}r$. So the fill is $4\mbox{ atoms}/a^3 = \frac{\sqrt{2}\pi}{6} = 74\%\;.$
For BCC, the most densely-packed plane is the inside diagonal, $\therefore \sqrt{3}a = 4\mbox{ radii} \therefore a = \frac{4}{\sqrt{2}} r$. So the fill is $2\mbox{ atoms}/a^3 = \frac{\sqrt{3}\pi}{8} = 68\%\;.$
If you find the FCC side length in terms of the atomic radius of copper, you will find that the answer is very close to the experimentally-found density of copper, $8920\textrm{ kg/m}^3.$
You may also like
2D-3D
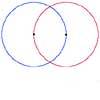
Two circles of equal size intersect and the centre of each circle is on the circumference of the other. What is the area of the intersection? Now imagine that the diagram represents two spheres of equal volume with the centre of each sphere on the surface of the other. What is the volume of intersection?
The Dodecahedron Explained
What is the shortest distance through the middle of a dodecahedron between the centres of two opposite faces?
When the Angles of a Triangle Don't Add up to 180 Degrees
This article outlines the underlying axioms of spherical geometry giving a simple proof that the sum of the angles of a triangle on the surface of a unit sphere is equal to pi plus the area of the triangle.

