Or search by topic
Number and algebra
Geometry and measure
Probability and statistics
Working mathematically
Advanced mathematics
For younger learners
Bent Out of Shape



- Problem
- Getting Started
If you are having trouble picturing how these molecule might look, try using blu tack and string to make models of the atoms and their bonds.
If you're still stuck, scroll down on this page to see diagrams of the molecules.
For the question about water, it will help you to think about the electrons in oxygen. How many valence electrons does oxygen have? What happens to the valence electrons that don't get used in bonding?
Structures of molecules given:
You will notice that in some of these diagrams some bonds are shown as normal lines, some as thick lines and some as dashed lines. This says nothing about the chemical nature of the bonds but describes their orientation in three dimensions. A normal line indicates a bond in the plane of the page (or screen); a thick line indicates a bond coming out of the page and a dashed line indicates a bond
going into the page. If this is confusing, don't worry! Try using these diagrams to help you make a model of the molecules or ask your teacher to explain further.
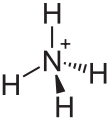 NH$_{4}^{+}$
NH$_{4}^{+}$
CH$_{4}$ has the same structure as NH$_{4}^{+}$
![]() BeH$_{2}$
BeH$_{2}$
AlCl$_{3}$ has the same structure as BF$_{3}$
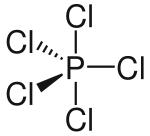 PCl$_{5}$
PCl$_{5}$
You may also like
Stats Statements
Are these statistical statements sometimes, always or never true? Or it is impossible to say?
Real-life Equations
Here are several equations from real life. Can you work out which measurements are possible from each equation?
A Question of Scale
Use your skill and knowledge to place various scientific lengths in order of size. Can you judge the length of objects with sizes ranging from 1 Angstrom to 1 million km with no wrong attempts?

