Skip over navigation
At a glance this initial part of the question appears exceptionally tricky. However, by breaking the simultaneous equilibrium into two separate equilibria, the problem is actually reasonably easy to analyse.
The first of these equilibria is:
$$H^+ + HCO_3^- + H_2O \rightleftharpoons^{K_1} H_2CO_3 + H_2O$$
From the original definition of K, it can be seen that:
$$K_1 = \frac{[H_2CO_3]}{[H^+][HCO_3^-]}$$
The second equilibrium is:
$$H_2CO_3 + H_2O \rightleftharpoons^{K_2} 2H_2O + CO_2$$
Again, from the definition of K, we can easily see that:
$$K_2 = \frac{[CO_2]}{[H_2CO_3]}$$
Eliminating $[H_2CO_3]$ from these gives:
$$ K_1[H^+][HCO_3^-] = \frac{[CO_2]}{K_2} $$
Rearranging:
$$ [H^+] = \frac{[CO_2]}{K_1K_2[HCO_3^-]}$$
Using the definition of pH allows:
$$ pH = -log_{10}\left(\frac{[CO_2]}{K_1K_2[HCO_3^-]}\right)$$
$$ = -log_{10}\left(\frac{1}{K_1K_2}\right) -log_{10}\left(\frac{[CO_2]}{[HCO_3^-]}\right)$$
$$ = -log_{10}\left(\frac{1}{K}\right) -log_{10}\left(\frac{[CO_2]}{[HCO_3]}\right)$$
$$ = pK -log\left(\frac{[CO_2]}{[HCO_3]}\right)$$
For the second part of this question, we are trying to prove that the inside of the logarithm is equivalent in each equation. Starting with the final equation and working in reverse:
$ \frac{1}{x} -1 -K_1[H^+]$
$ = \left(\frac{[HCO_3^-] + [H_2CO_3] + [CO_2]}{[HCO_3^-]}\right)\ -1\ -\left(\frac{[H^+][H_2CO_3]}{[H^+][HCO_3^-]}\right)$
$ = \left(\frac{[H_2CO_3]}{[HCO_3^-]}\right) + \left(\frac{[CO_2]}{[HCO_3^-]}\right) - \left(\frac{[H_2CO_3]}{[HCO_3^-]}\right)$
$= \left(\frac{[CO_2]}{[HCO_3^-]}\right)$
This is as required.
By treating $K_1[H^+]$ as very small, we can neglect it, and as the pH expression becomes:
$$pH = 6.1 - log( \frac{1}{x} -1)$$
$$ = 6.1 - log\left(\frac{1-x}{x}\right)$$
Plotting this gives the required buffer curve:
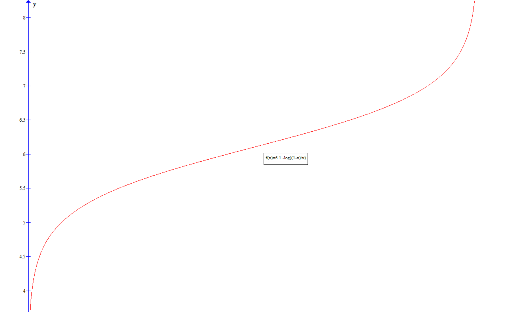
Extension:
1) We are dealing with pHs in the region of 4- 8, and so the $[H^+] = 10^{-4} to 10^{-8}$. This is very small compared to the $\frac{1}{x} -1$ term, and so can be neglected. Additionally, because this quantity is subsequently logged, such a small difference is made even smaller, such that it can certainly be neglected.
2) This part of the problem requires a solid grip on the chain rule, and logarithmic manipulation. It is solved as shown:
$$pH = 6.1 - log\left(\frac{1-x}{x}\right)$$
$$ = 6.1 - \frac{ln\left(\frac{1-x}{x}\right)}{ln(10)}$$
$$ = 6.1 - \frac{1}{ln(10)}\left(ln(1-x) -ln(x)\right)$$
$$\therefore \frac{d(pH)}{dx}= \frac{1}{ln(10)} \left(\frac{1}{1-x} + \frac{1}{x}\right)$$
$$\therefore \frac{d^2(pH)}{dx^2} = \frac{1}{ln(10)} \left(\frac{1}{(1-x)^2} -\frac{1}{x^2}\right) = 0$$
$$ \frac{1}{(1-x)^2} = \frac{1}{x^2}$$
$$ x =0.5$$
Thus the second derivative of the pH is zero when x = 0.5. This corresponds to a point of inflection on the graph, which is where the gradient of the gradent is zero: at this point the curve ceases to decrease in gradient and begins to again increase in gradient.


Or search by topic
Number and algebra
Geometry and measure
Probability and statistics
Working mathematically
Advanced mathematics
For younger learners
Blood Buffers
Age 16 to 18
Challenge Level 





- Problem
- Getting Started
- Student Solutions
- Teachers' Resources
At a glance this initial part of the question appears exceptionally tricky. However, by breaking the simultaneous equilibrium into two separate equilibria, the problem is actually reasonably easy to analyse.
The first of these equilibria is:
$$H^+ + HCO_3^- + H_2O \rightleftharpoons^{K_1} H_2CO_3 + H_2O$$
From the original definition of K, it can be seen that:
$$K_1 = \frac{[H_2CO_3]}{[H^+][HCO_3^-]}$$
The second equilibrium is:
$$H_2CO_3 + H_2O \rightleftharpoons^{K_2} 2H_2O + CO_2$$
Again, from the definition of K, we can easily see that:
$$K_2 = \frac{[CO_2]}{[H_2CO_3]}$$
Eliminating $[H_2CO_3]$ from these gives:
$$ K_1[H^+][HCO_3^-] = \frac{[CO_2]}{K_2} $$
Rearranging:
$$ [H^+] = \frac{[CO_2]}{K_1K_2[HCO_3^-]}$$
Using the definition of pH allows:
$$ pH = -log_{10}\left(\frac{[CO_2]}{K_1K_2[HCO_3^-]}\right)$$
$$ = -log_{10}\left(\frac{1}{K_1K_2}\right) -log_{10}\left(\frac{[CO_2]}{[HCO_3^-]}\right)$$
$$ = -log_{10}\left(\frac{1}{K}\right) -log_{10}\left(\frac{[CO_2]}{[HCO_3]}\right)$$
$$ = pK -log\left(\frac{[CO_2]}{[HCO_3]}\right)$$
For the second part of this question, we are trying to prove that the inside of the logarithm is equivalent in each equation. Starting with the final equation and working in reverse:
$ \frac{1}{x} -1 -K_1[H^+]$
$ = \left(\frac{[HCO_3^-] + [H_2CO_3] + [CO_2]}{[HCO_3^-]}\right)\ -1\ -\left(\frac{[H^+][H_2CO_3]}{[H^+][HCO_3^-]}\right)$
$ = \left(\frac{[H_2CO_3]}{[HCO_3^-]}\right) + \left(\frac{[CO_2]}{[HCO_3^-]}\right) - \left(\frac{[H_2CO_3]}{[HCO_3^-]}\right)$
$= \left(\frac{[CO_2]}{[HCO_3^-]}\right)$
This is as required.
By treating $K_1[H^+]$ as very small, we can neglect it, and as the pH expression becomes:
$$pH = 6.1 - log( \frac{1}{x} -1)$$
$$ = 6.1 - log\left(\frac{1-x}{x}\right)$$
Plotting this gives the required buffer curve:

Extension:
1) We are dealing with pHs in the region of 4- 8, and so the $[H^+] = 10^{-4} to 10^{-8}$. This is very small compared to the $\frac{1}{x} -1$ term, and so can be neglected. Additionally, because this quantity is subsequently logged, such a small difference is made even smaller, such that it can certainly be neglected.
2) This part of the problem requires a solid grip on the chain rule, and logarithmic manipulation. It is solved as shown:
$$pH = 6.1 - log\left(\frac{1-x}{x}\right)$$
$$ = 6.1 - \frac{ln\left(\frac{1-x}{x}\right)}{ln(10)}$$
$$ = 6.1 - \frac{1}{ln(10)}\left(ln(1-x) -ln(x)\right)$$
$$\therefore \frac{d(pH)}{dx}= \frac{1}{ln(10)} \left(\frac{1}{1-x} + \frac{1}{x}\right)$$
$$\therefore \frac{d^2(pH)}{dx^2} = \frac{1}{ln(10)} \left(\frac{1}{(1-x)^2} -\frac{1}{x^2}\right) = 0$$
$$ \frac{1}{(1-x)^2} = \frac{1}{x^2}$$
$$ x =0.5$$
Thus the second derivative of the pH is zero when x = 0.5. This corresponds to a point of inflection on the graph, which is where the gradient of the gradent is zero: at this point the curve ceases to decrease in gradient and begins to again increase in gradient.
You may also like
bioNRICH
bioNRICH is the area of the stemNRICH site devoted to the mathematics underlying the study of the biological sciences, designed to help develop the mathematics required to get the most from your study of biology at A-level and university.
Catalyse That!
Can you work out how to produce the right amount of chemical in a temperature-dependent reaction?

